Flow Programming for Batch Measurement
By stopping the flow, while reaction mixture is within the flow cell, the dispersion of reacting components is halted, and the monitored response is due only to reaction kinetics. However, in order to perform measurement in batch mode, the also the following conditions must be met:
- Sample has to be mixed with reagents in known, and well defined ratio.
- The flow cell has to be entirely filled with homogenous mixture of reactants
- The reaction mixture must be held within the flow cell until chemical equilibrium is reached.
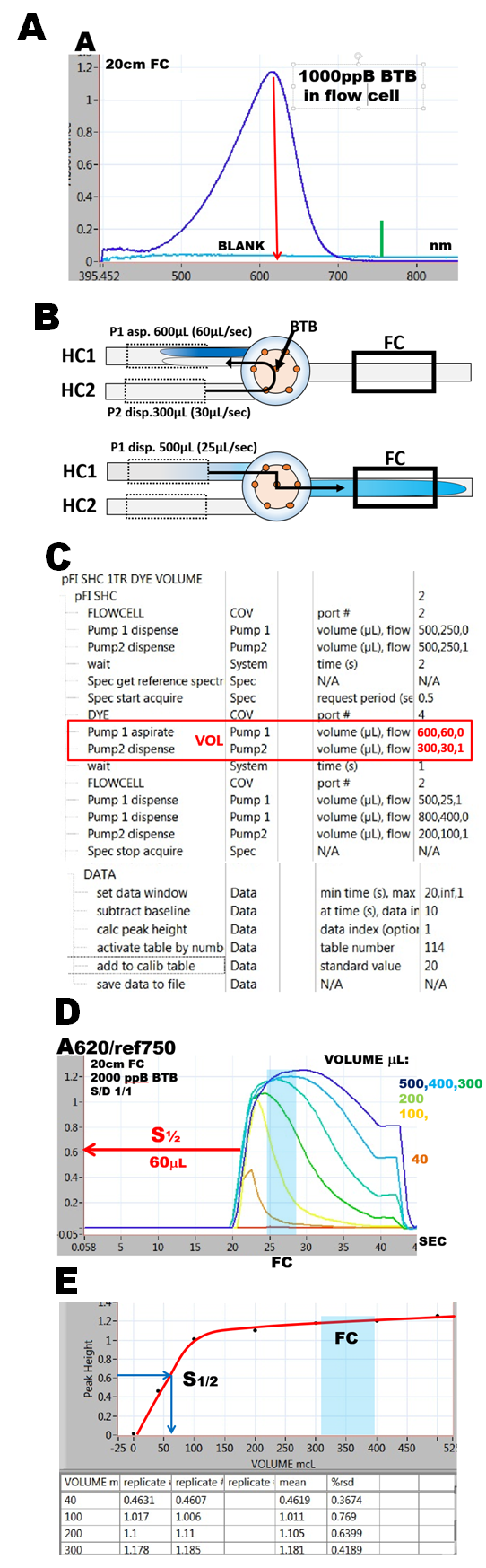
Deprotonated form of bromothymol blue (BTB) was used to find, how the above requirements can be fulfilled with acceptable reproducibility and accuracy. The apparatus used was miniSI-2, equipped with 20 cm long flow cell. Monitoring of absorbance was performed at 620nm with reference wavelength at 750nm (A).
Mixing of BTB (buffered at pH 11) with carrier (DI water) was controlled by flowrates and volumes delivered to the confluence point by miliGAT pumps via holding coils (HC1 and HC2) (B). The software protocol (C) was designed to deliver into and through the flow cell a mixture composed of equal volumes of BTB and DI water used as diluent (VOL).
Series of experiments where the combined volumes were increased from 40 to 500 mcrL (D, E) revealed that 200mcrL of BTB diluted with equal volume of DI fills the flow cell (volume 100mcrL – blue rectangle in D, E) with solution, the optical density of which is homogenous throughout the optical path ( 20 cm long) and corresponds to 2000ppB BTB diluted 1:1. Relation between absorbance and volume of BTB follows the well established exponential relationship (0.3.3., 1.2.12.) characterized by S1/2 value. For all following experiments the volume of reaction mixture (VOL) was chosen to be 400mcrL, because this volume, being much larger than volume of the flow cell, will leave the front and tailing edges, diluted by surrounding carrier stream, outside of 100mcrL volume of the light path (D, E). Also because volume of 400mcrL is close to 6 x S1/2 the, monitored absorbance will be within 1% of maximum achievable absorbance value (0.3.3., 1.2.12.).
1.5.2.